Trick or Treat
You will start working on the cell analogy project. You will work in groups of two to compare the activities of the organelles of the cell to a factory, a city, or any other organization. Choose something you know.
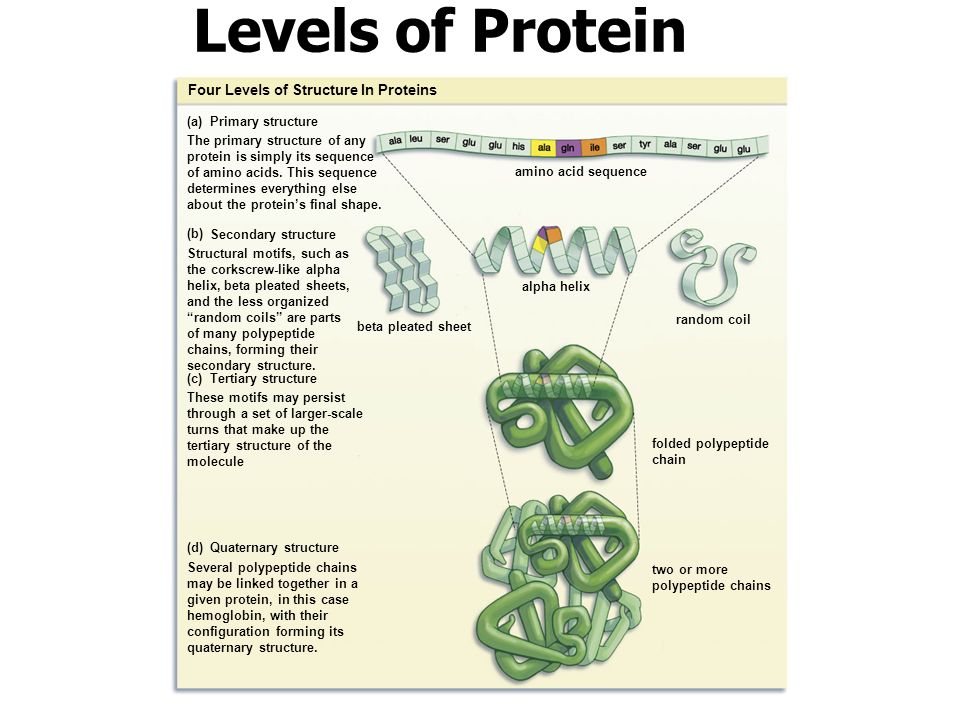
For your project you will describe the function of the first 14 organelles and then describe the comparison and state why the function of the analogy is similar to the organelle.
Draw pictures of the analogy.
Include the organelles discussed in class yesterday excepting the cell wall, the chloroplast.
The grade for this assignment will be included as a project grade.